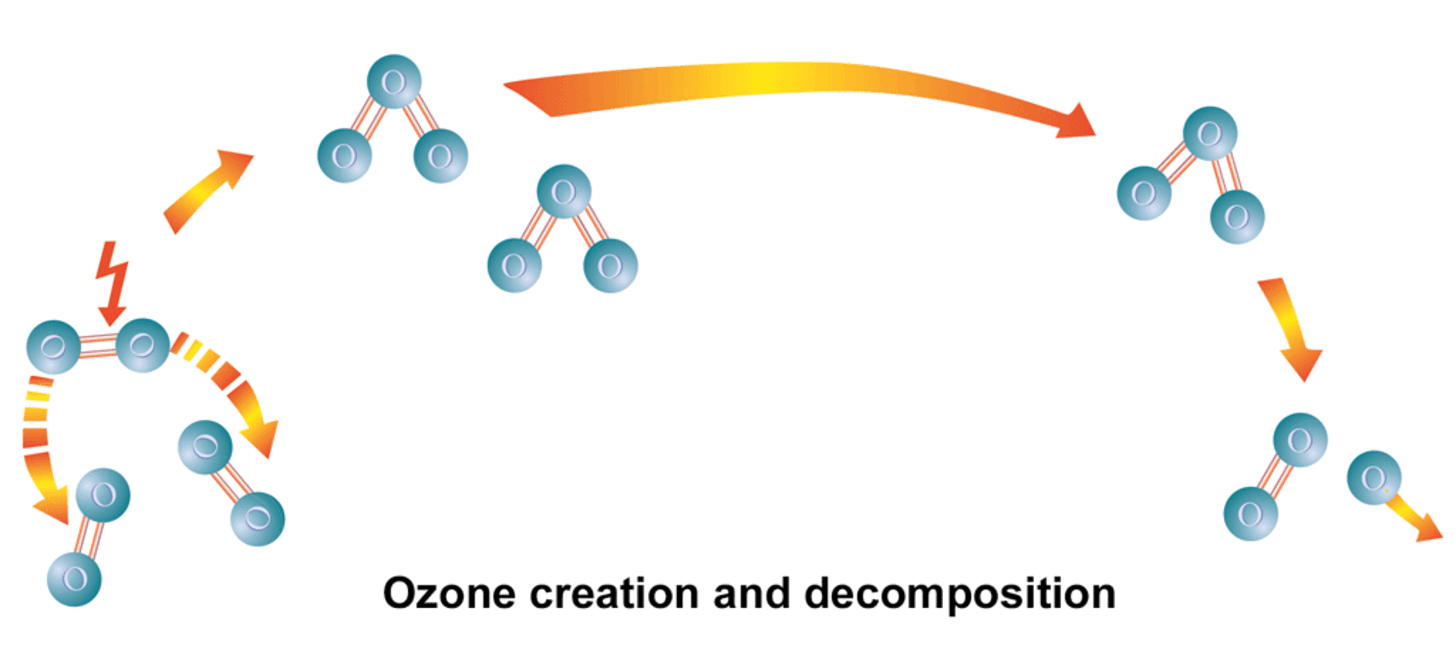
What is ozone?
Ozone (O₃) is the triatomic form of oxygen. It is unstable and therefore highly reactive. This is why ozone is often described as activated oxygen. This unstable bond makes ozone a strong oxidizing agent with a high specific oxidation potential (2.07 V). When it comes into contact with organic or inorganic compounds, an oxygen atom reacts in an oxidative reaction and the ozone breaks down into molecular, stable oxygen (O₂).
How is ozone formed
Natural ozone
In nature, ozone occurs in higher concentrations almost exclusively in the stratosphere. There, it is continuously formed by the high-energy, short-wave UVC radiation from the sun.
Technically produced ozone
For industrial and technical applications, ozone is generated artificially using ozone generators. The underlying principle is based on a silent electrical discharge (also known as corona discharge or glow discharge), which occurs through ionization in a non-conductive medium.
A typical ozone generator consists of:
- at least two flat high-voltage electrodes
- a dielectric barrier between them (e.g. glass or ceramic)
- a power supply unit with a high-voltage generator
The process gas – usually dry air or pure oxygen – flows through the alternating high-voltage field. The electric field strength in the discharge gap ionizes the gas molecules and provides the activation energy to split oxygen molecules (O₂) into individual oxygen atoms (O). These highly reactive atoms then combine with other O₂ molecules to form ozone (O₃).
Ozone concentration and production output
Only a portion of the oxygen fed into the system will actually be converted into ozone. The ozone concentration in the gas mixture produced is usually specified in grams of ozone per standard cubic meter (g/Nm³) or as a percentage by weight (% wt).
In combination with the gas volume flow, this can be used to calculate the production output of an ozone generation system.
Typical ozone concentrations depending of the process gas:
| Process gas: | Ozon concentration |
|---|---|
| Dry air | up to 30 g/Nm³ or 2 % (wt) |
| Oxygen from PSA systems | up to 150 g/Nm³ or 10 % (wt) |
| Pure oxygen from LOX | up to 300 g/Nm³ or 20 % (wt) |
Concentrations of 7 to 10% (wt) are common in water treatment.
How does ozonation work?
Ozone reliably eliminates bacteria, viruses, and other microorganisms. In addition, inorganic substances are oxidized and organic compounds are broken down into smaller, biodegradable components through oxidation.
These low-molecular compounds – known as assimilable organic carbon (AOC) – are then further broken down and mineralized in a biologically active filter bed (ozone biofiltration).
Advantages of Ozonation
- Reliable oxidation of inorganic substances such as iron, manganese, and arsenic
- Effective disinfection without harmful residues
- Decomposition of complex organic compounds
- Reduction of odors, taste disturbances, and chemical residues
- Environmentally friendly alternative to chlorination – no THM formation
Processes Involved in Ozonation
The following processes typically occur during ozonation:
- Disinfection of raw water
- Oxidation of iron to filterable iron hydroxide
- Oxidation of manganese to filterable manganese dioxide
- Oxidation of arsenic to filterable arsenate
- Breakdown of high-molecular organic compounds to support biological mineralization in the filter bed (ozone biofiltration)
- Oxygen enrichment to support aerobic microbiology in the biologically active filter bed
Is Ozonation the Right Solution for Your Water Treatment Challenge?
Are you unsure whether ozonation is suitable for your specific application – or how it could be integrated into your existing treatment system?
We're here to help.
Tell us about your requirements, and we’ll assist you with:
- a technical evaluation of your case,
- selecting the most effective process, and
- the design and dimensioning of a tailored ozone solution.
Benefit from our expertise and hands-on experience in practical system integration.