The degassing solution
Special degassing methods can therefore be used to remove gases from the water or to reduce them to below the respective required residual concentrations (guaranteed value).
The guaranteed value is defined as the remaining quantity of oxygen or carbon dioxide that continually reaches or undershoots degassing.
Thermal degassing
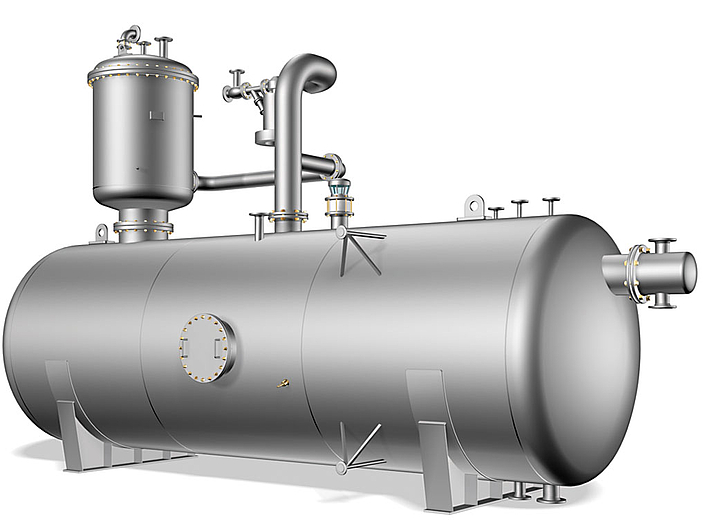
Thermal degassing (pressure degassing) of boiler feedwater requires that the water be boiling. The mixture of condensate and fresh water is fed into the degassing dome at the top. Heating up to the boiling temperature occurs with heating steam, which flows into the degassing unit at the bottom and flows upward against the current. The gases driven out as a result are lead away with the exhaust vapours via the exhaust vapour outlet in the degassing dome. The degassed water is collected in the feedwater tank.
Thermal degassing occurs at temperatures just above 100°C and operating pressures around 0.2 to 0.3 bar. Thus an oxygen content under 0.02 mg O2/l and a carbon dioxide content of less than 1 mg CO2/l are possible in the feedwater.
With vacuum degassing, water can also be degassed below 100°C by boiling. Creating a corresponding negative pressure (partial vacuum) in the degassing unit enables the boiling point to be reached as early as in the 30-80 °C range. Here degassing takes place in a percolator with a spraying unit, packing and supply section. The gases are removed via a vacuum pump with the resulting water vapour. It is preferable to use an exhaust vapour condenser to enable the vacuum pump's intake volume flow to be kept low. The residual oxygen content of 0.02 mg/l can also be achieved here.
Vacuum degassing is used for treating supplementary water for heating systems.
Exhaust vapour condenser
A high part of the thermal energy of the vapours can be recovered by means of a vapour condenser. This thermal energy is usually used for heating additional water, which leads to a reduced heating steam requirement. For thermal pressure degassing, this condenser is not absolutely necessary, but is recommended for economic reasons above a defined size of the degassing system. In vacuum degassing, the vapour condenser is particularly important for the economical dimensioning of the vacuum pump.
Membrane degassing
Membrane degassing enables dissolved carbon dioxide (CO2) to be removed from the water in pure stripping air mode down to values of < 2 mg/l (ppm) or, in combination with a vacuum pump, down to values of < 1 mg/l (ppm). Using industrial nitrogen as the stripping medium and in combination with a vacuum pump enables oxygen to be reduced to residual values of < 1 µg/l (ppb).
Creating a corresponding partial pressure gradient as the driving force (stripping with air/gas and/or creating a vacuum) causes the gases to diffuse out of the liquid and into the gas-bearing membrane fibres through microporous, hydrophobic (water-impermeable) hollow-fibre membrane modules, and to be transported away with the stripping gas.
The individual hollow-fibre membranes are combined in modules (known as membrane contactors or as membrane degassing units = MDU).
Membrane degassing is used to support desalination processes. In the case of demineralization with ion exchangers, membrane degassing allows the load to be relieved on the anion exchanger, resulting in a significant reduction in the need for regeneration chemicals (mainly sodium hydroxide). This makes membrane degassing a good alternative to the CO2 percolator, but with the advantages of requiring much less space and energy (e.g. no pressure increase required).
In addition, membrane degassing is useful between EDI (electrode ionisation) and reverse osmosis.
Chemical degassing
Chemical degassing or subsequent degassing occurs through the addition of chemicals. These chemicals are dissolved in dilution water (minimum soft water quality) and are suitable for removing or converting the oxygen contained in the water. The reaction time differs when different chemicals are used. In general, the greater the water temperature, the shorter the reaction time.
Due to the heavy use of chemicals, chemical degassing is of interest only at low boiler pressures and a low system performance. In medium-sized and large systems, chemical degassing is used only when it is necessary to achieve residual oxygen contents of < 10 µg/l.
More detailed information you will find in the download area.
Or even better: Call us, we will be happy to advise you!