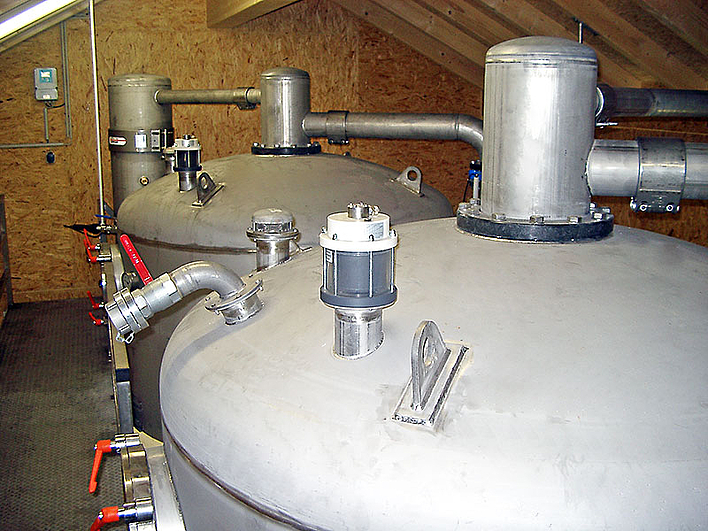
Plants for chemical and physical deacidification
Natural groundwater or spring water often has an excessive amount of carbon dioxide, which must be removed by technical treatment measures. Deacidification is also necessary after treatment steps such as nanofiltration or reverse osmosis.
The choice of the optimum deacidification process depends on the raw water quality, the treatment objective and the desired water quality:
Chemical deacidification
By filtration over materials containing calcium carbonate (semi-fired dolomite, marble or jurassic lime), the free aggressive carbonic acid is neutralised to the lime-carbonic acid balance. The free carbonic acid dissolves calcium carbonate from the filter material, which must be gradually used up and regularly refilled. To facilitate this process, larger deacidification plants are equipped with systems for automatic or semi-automatic refilling of the filters with deacidification material.
Chemical deacidification leads to an increase in water hardness. For this reason, deacidification filters should only be used in very soft waters to increase the alkalinity in the range of the degrees of hardness "soft".
Underdimensioned limestone filtration
For soft waters with very high carbon dioxide contents, complete chemical deacidification would lead to large filters and a comparatively high increase in hardness. In order to avoid this, so-called under-dimensioned limestone filtration is often used in these cases.
With this process, the deacidification filters are designed only so large that the desired target hardness is achieved. The CO2, which is not bound chemically, will then be removed from the water by means of physical deacidification. In this process, the systems must be operated under nominal load in order to avoid greater fluctuations in water quality. Alternatively, the systems can be equipped with a bypass control systems.
Aluminium removal
By combination of two filters in series with controlled partial flow return, aluminium can be reliably removed from the water by means of chemical deacidification. In the first filter, the pH value is regulated to the value required for aluminium precipitation The precipitated aluminium accumulates in the filter and is then removed from the filter by backwashing. This ensures long-term stable deacidification with aluminium removal.
Physical deacidification
Physical or mechanical deacidification uses the property of water that dissolved gases can be expelled from the water by mechanical energy input and partial pressure differences. The ideal process depends on the amount of gas to be removed.
The following processes are used:
- Spraying
- Sprinkling via cascades or tricklers
- Cross-flow aeration in flat bed
Spraying and Trickler
In many cases, sufficient residual degassing is possible by means of evaporation via special nozzles. Radon and hydrogen sulphide can also be efficiently removed from the water by evaporation. The evaporation can take place directly in the tank. Appropriate ventilation and exhaust air ducting is required for the gas discharge. Both uniflow and counterflow versions are possible.
Rieslers are closed containers, which are often still equipped with water distribution systems or packing. As a rule, operation takes place in counterflow. The tanks can be made of PE or stainless steel.
Suitable filters and thermally insulated pipes are required in all cases for the supply air and exhaust air ducting to prevent condensation on the surfaces.
To select the right system, information on the water flow rate and the composition of the water to be treated is required.
Cross-flow aeration
Cross-flow aerators or flatbed aerators are one of the most effective deacidification processes. They are used in particular for high gas contents. The water flow, which is guided horizontally in a flat container, is blown with air in a vertical direction. Tubes made of a sintered material are used for air intake. High deacidification capacities are achieved with low energy consumption due to the fine-bubble air input via the ceramic aeration pipes. CO2 can thus be removed down to residual contents of 1.5 mg/l (pH value 7.7; calcite dissolving capacity <5 mg/l).
The deacidification capacity can be easily adapted to different throughput rates by changing the air quantities.