Demanganisation
The limit value for manganese in drinking water is 0.05 mg/l. This low value is not based on health considerations but primarily serves to protect the distribution network from silting and the formation of black or brown manganese oxide deposits. Manganese often occurs together with iron in water.
Water containing manganese is generally reduced-oxygen or low-oxygen water in which the manganese is present dissolved in bivalent form as Mn2+. Manganese can be removed almost completely from water through demanganisation.
Like iron, manganese is a heavy metal that is normally only present as an oxide in the earth's crust. During the course of leaching, water, the oxygen content of which decreases over time due to biological processes, comes into contact with these oxides in deeper layers of the earth. If the oxygen content is low, redox processes in the soil reduce oxides and the manganese released in the process is dissolved in water. Oxidized manganese forms black deposits in pipes, which can cause massive problems.
Processes for demanganisation
Like deferrization, demanganization is one of the oldest water treatment processes along with filtration. In demanganization, the soluble divalent manganese is oxidized with oxygen to form insoluble manganese oxide (manganese dioxide). Pure demanganization is not as easy as deferrization, because manganese is only oxidized after iron. In classic treatment by means of aeration, two filter stages are therefore usually connected in series. Deferrization takes place in the first stage, with demanganization taking place in the second stage.
As described in the section on deferrization, oxygen enrichment must first be ensured for all of the processes described below.
Biological demanganization
Biological demanganisation can require running-in periods of several months. In addition to a sufficiently high oxygen content, a stable pH value is necessary. By inoculating with biologically wetted filter sand from a functioning filtration stage, the running-in time of the inert filter material can be significantly reduced. In addition to biological processes, catalytic effects also occur in demanganisation filters. For this reason, well-established demanganisation filters operate quite stably.
Catalytic demanganization
With special catalytically-active filter materials and depending on the manganese content of the raw water, reliable demanganization can also be carried out with one or two filter stages. Catalytic demanganization requires high redox potentials, which is why potassium permanganate dosing is often used. Materials with a catalytic effect are often used in process water treatment. The materials can lose their catalytic properties over time and their effectiveness may deteriorate.
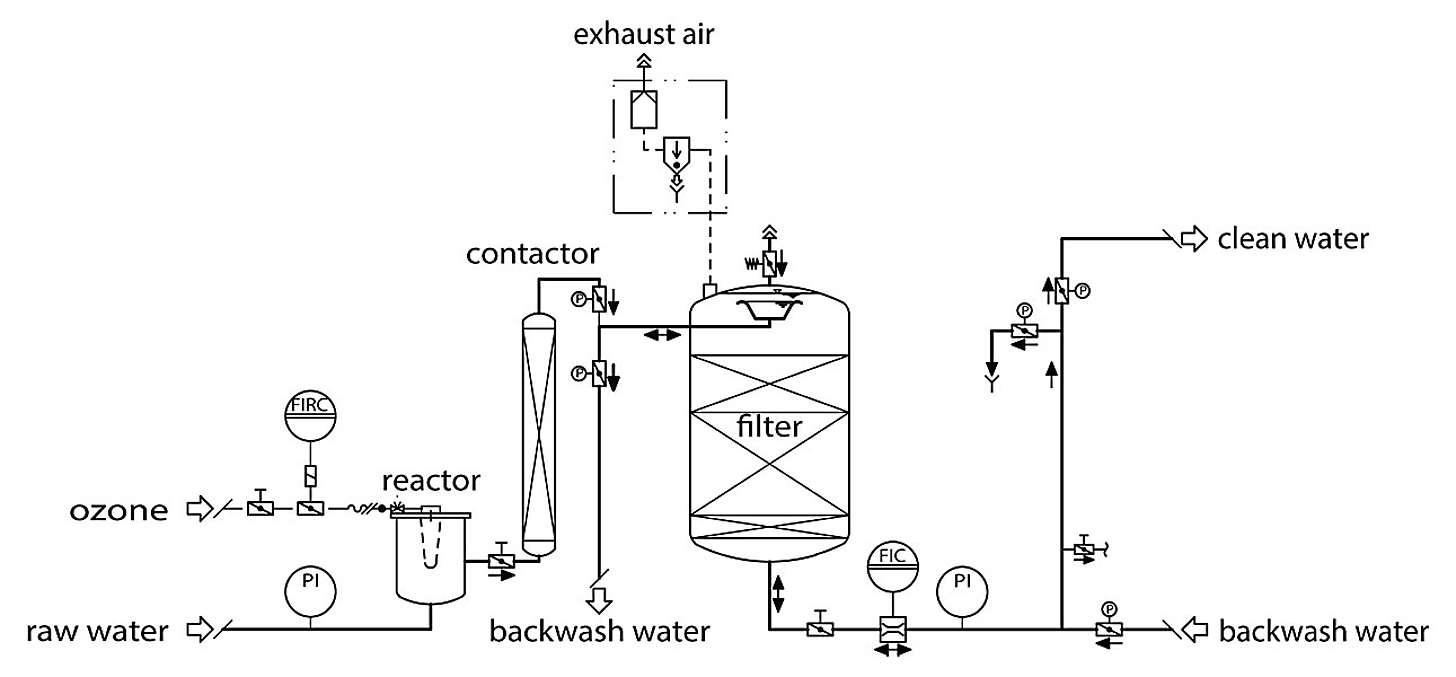
Chemical demanganization
When using ozone, manganese can be removed in a filter stage together with iron and any arsenic that may be present. The advantage of ozone oxidation is complete demanganization without an inlet time. When ozoning however, it must be noted that permanganate can form if the ozone dose is too high, thus giving the water a purple colour.
Demanganization can also occur by adding permanganate. However, dosing requires a constant manganese content in the raw water, which is often not the case. For this reason, we favour ozoning.