Iron Removal
Drinking water with an iron content of more than 0.2 mg/l must be treated, even if iron does not pose an immediate health risk.
Iron impairs the sensory quality of drinking water - in particular taste, color, and clarity - and causes technical problems such as pipe rusting and sludge formation due to sedimentation.
Iron is a heavy metal that occurs primarily in oxidized form as iron oxide (Fe₂O₃) in the Earth's crust.
As water percolates into deeper layers of soil, its oxygen content gradually decreases due to biological activity. In oxygen-depleted zones, the water comes into contact with iron oxides. Microbial metabolic processes then remove oxygen from the iron oxides, releasing iron into the water in a dissolved, divalent form (Fe²⁺).
Although dissolved iron in oxygen-poor water is physiologically harmless, it presents significant technical challenges: Even low concentrations can lead to deposits and incrustations in pipes, fittings (e.g., aerators), and household appliances such as washing machines. In addition, iron-oxidizing microorganisms like Gallionella can cause further problems. These bacteria use the energy released during the oxidation of Fe²⁺ to Fe³⁺ as a source of energy and, under favorable conditions, form slimy, gelatinous masses that can cause serious operational disruptions.
For these reasons, iron removal is among the oldest and most essential process in water treatment, alongside filtration.
Processes for deferrization
Deferrization generally requires oxidation, in which dissolved iron(II) (Fe²⁺) is converted to iron(III) (Fe³⁺).
Biological deferrization
As outlined above, biological deferrization plays little to no role on a technical scale and occurs mainly in natural environments. Maintaining a stable process demands strict control of key parameters, including low and stable oxygen concentrations, appropriate pH, balanced CO₂ levels, and a defined redox potential. Because of these stringent requirements—and the availability of more efficient alternatives—this method is now largely irrelevant in modern drinking water treatment.
Chemical deferrization
Chemical deferrization occurs through simple oxidation with oxygen. The literature contains a wide variety of information about reaction speeds, most of which does not correspond to practical experience. The fact is that divalent iron dissolved in water oxidizes very quickly with oxygen to form insoluble trivalent iron oxide hydrate FeO(OH). The crucial variable for oxidation is the oxygen content in the water, i.e. the gaseous oxygen must first be dissolved in the liquid water.
This means that the oxygen content and the gas introduction and mixing system are process-relevant variables.
Oxidation with atmospheric oxygen
If the iron content is low, air is mixed with the raw water upstream of the filter to oxidize the iron. However, air contains only about 21 % oxygen and 78% nitrogen. For this reason, high air volumes, large reaction vessels (oxidizers) and large ventilation systems are required for higher iron contents.
We therefore only recommend oxidation with atmospheric oxygen for smaller systems and low iron contents.
Oxidation with oxygen
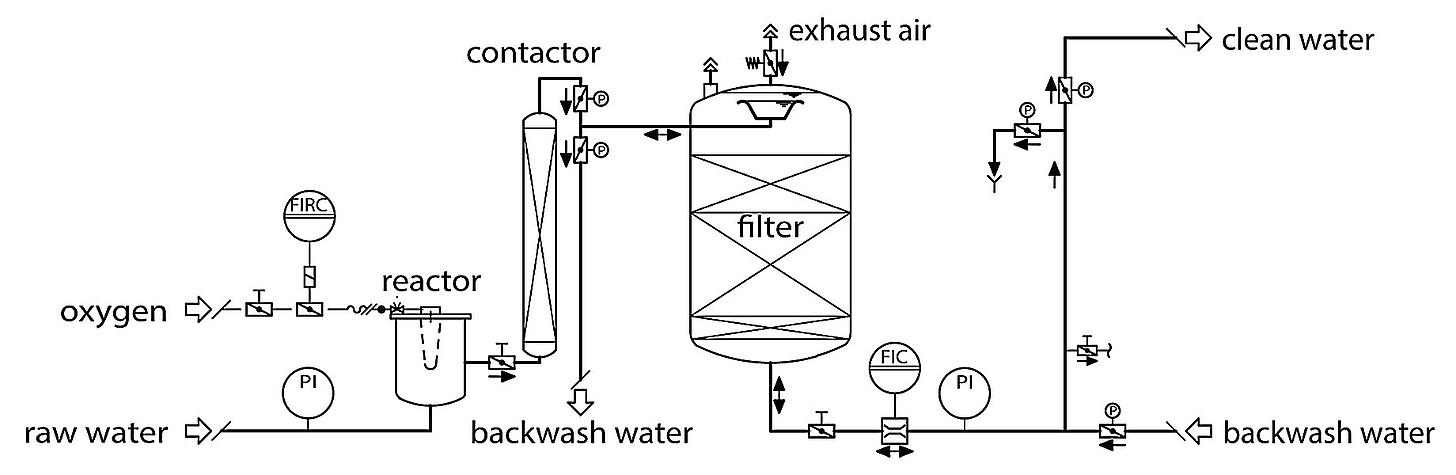
Atmospheric oxygen can be concentrated to 95% with oxygen generators. At just under 5%, nitrogen no longer plays a disturbing role. The venturi / injector systems allow the oxygen to be dissolved in the water very efficiently. The oxidation of the iron proceeds very quickly and does not require large reaction vessels. The retained iron oxide hydrate forms good flakes, making it very easy to separate and, over time, results in an increase in the differential pressure in the filter. Back-flushing generally occurs as a function of the load and therefore the throughput. Extremely effective deferrization (< 0.01 mg/l) can be achieved in particular even with high iron contents in the raw water by way of classic multi-layer filtration with upstream oxygenation. The use of membrane systems (microfiltration / ultrafiltration) for deferrization can be considered for smaller iron contents.
Oxidation with ozone
In the case of organically-bound iron, ozone might be necessary for oxidation. As a strong oxidizing agent, ozone breaks down humic bridges and can thereby also oxidize iron.
We would be happy to assist you with the dimensioning of a water treatment system and with finding the optimum configuration.
Please contact us!